Lot traceability forms help track product batches throughout the supply chain, ensuring quality control and regulatory compliance. These forms typically record information like batch numbers, production dates, and distribution details to facilitate efficient recall processes. Accurate lot traceability is essential for industries such as food, pharmaceuticals, and manufacturing to maintain transparency and consumer safety.
Lot Traceability Form Sample PDF Viewer
Image example of Lot Traceability Form:
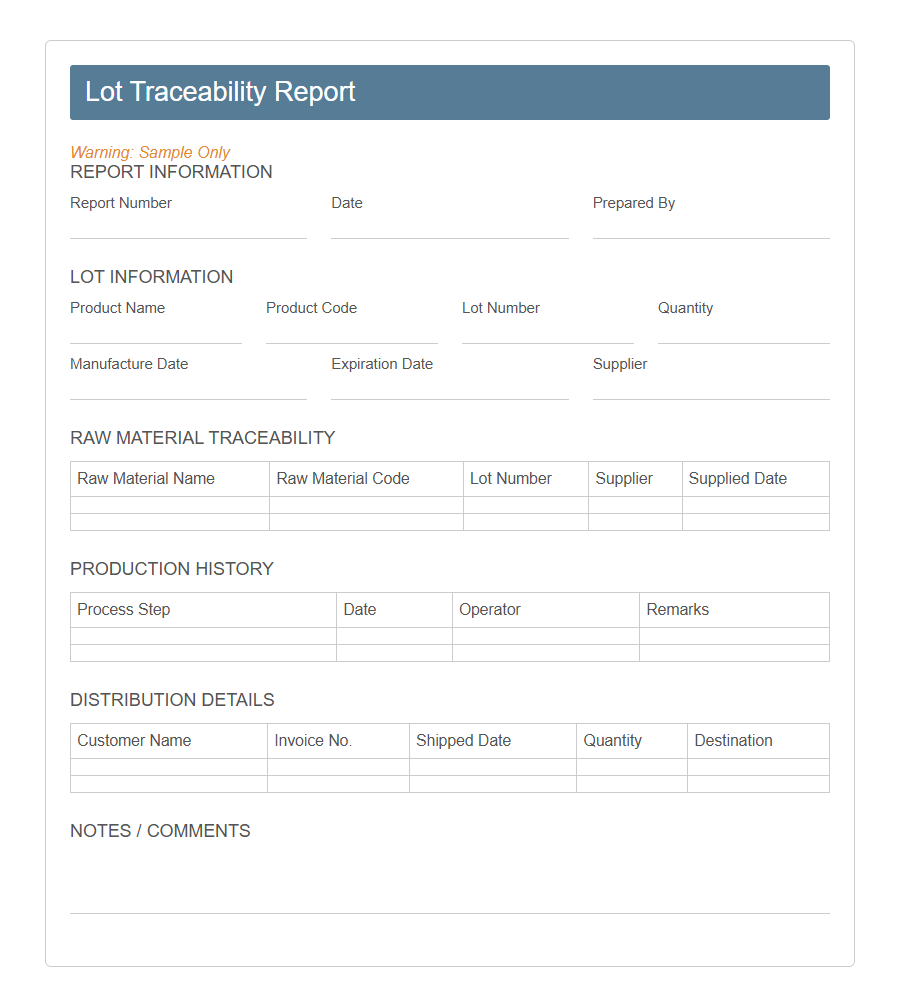
Lot Traceability Form Samples
Lot Traceability Report Template - PDF - HTML
Batch Tracking Log Sheet - PDF - HTML
Product Lot Identification Form - PDF - HTML
Lot Number Registration Sheet - PDF - HTML
Manufacturing Lot Traceability Record - PDF - HTML
Warehouse Lot Tracking Document - PDF - HTML
Lot Recall Traceability Template - PDF - HTML
Incoming Material Lot Tracking Form - PDF - HTML
Finished Goods Lot Control Sheet - PDF - HTML
Food Production Lot Traceability Form - PDF - HTML
Supplier Lot Certification Template - PDF - HTML
Lot Traceability Audit Checklist - PDF - HTML
Pharmaceutical Lot Records Sheet - PDF - HTML
Introduction to Lot Traceability
Lot traceability is a crucial process in manufacturing and supply chain management that tracks the history, application, and location of a product batch. It ensures quality control by enabling the identification of specific lots in case of defects or recalls. Effective lot traceability enhances transparency, accountability, and regulatory compliance throughout the production cycle.
Importance of Lot Traceability in Quality Control
Lot traceability is essential in quality control as it allows manufacturers to track and identify products throughout the production process. This capability helps quickly isolate and address defects, minimizing potential risks to consumers and reducing costs associated with recalls. Effective lot traceability ensures compliance with industry standards and enhances overall product safety and reliability.
Key Elements of a Lot Traceability Form
A Lot Traceability Form is essential for tracking products through the supply chain to ensure quality and safety. It helps businesses quickly identify and isolate issues related to specific batches or lots.
- Lot Number - A unique identifier assigned to each batch to distinguish it from others in production and distribution.
- Production Date - The specific date when the lot was manufactured, aiding in tracking and expiry management.
- Source and Destination Details - Information on the origin and intended recipient to monitor the movement of the lot through the supply chain.
This form is crucial for maintaining product integrity and facilitating efficient recalls if necessary.
How to Create an Effective Lot Traceability Form
Creating an effective Lot Traceability Form is essential for tracking products through the supply chain and ensuring quality control. A well-designed form streamlines recall processes and enhances inventory management efficiency.
- Include Clear Identification Fields - Use unique lot numbers and batch details to ensure accurate product tracking throughout the production and distribution stages.
- Incorporate Comprehensive Data Sections - Capture key information such as production date, supplier details, and quantity to maintain a complete record for quality assurance.
- Design User-Friendly Layouts - Organize the form logically with clear labels and spaces to minimize errors and facilitate quick data entry by staff.
Essential Data Fields in Lot Traceability Documentation
The Lot Traceability Form is vital for tracking product batches throughout the supply chain. It ensures accurate identification and retrieval of information related to production and distribution.
Essential data fields include lot number, production date, expiration date, and supplier details.
Compliance Standards for Lot Traceability
Lot Traceability Forms are essential tools for maintaining detailed records of product batches throughout the supply chain.
These forms ensure that each lot can be accurately tracked from origin to final distribution, supporting effective quality control and recall processes. Compliance with established standards is critical to guarantee data integrity and traceability accuracy.
Best Practices for Managing Lot Traceability Forms
Effective management of Lot Traceability Forms is crucial for ensuring product quality and regulatory compliance. Proper handling of these forms enhances traceability and facilitates efficient recall processes when necessary.
- Standardize Form Design - Use clear, consistent formats to reduce errors and improve data recording accuracy across all batches.
- Implement Digital Tracking - Utilize electronic systems to store and manage lot data for faster access and improved security.
- Train Staff Regularly - Provide ongoing education to employees on accurate form completion and the importance of traceability.
Digital vs. Paper Lot Traceability Forms
How does digital lot traceability compare to paper lot traceability forms? Digital lot traceability forms offer faster data entry and easier access to historical records. Paper forms can be prone to errors and are harder to store and retrieve efficiently.
Common Challenges in Lot Traceability Recordkeeping
Lot Traceability Forms are essential for maintaining accurate records in production and inventory management.
Common challenges in lot traceability recordkeeping include inconsistent data entry and lack of standardization across departments. These issues often lead to difficulties in tracking product history and recalling defective batches efficiently.
